Diagnostics for infectious and malignant diseases often exhibit poor specificity/sensitivity, hindering early detection and treatment evaluation, but development of improved assays is limited by several challenges, including absence of disease-specific factors, low biomarker concentrations, and interfering factors. Our center aims to develop novel strategies to overcome these obstacles, exploiting proteomic information and nanomaterial properties to generate integrated assay platforms for personalized medicine.
Platform I.
Ultra-sensitive Detection of SARS-CoV-2
Our research on COVID-19 has been devoted to developing more sensitive assays that to improve diagnosis, screening, and treatment evaluation efforts. Improved assay methods are urgently needed since current assays are known to miss significant numbers of cases and appear to falsely identify viral clearance in a subset of patients that subsequently develop disease recurrence. My research team has developed a rapid, ultrasensitive COVID-19 diagnostic assay that is more sensitive, and requires less specialized equipment, than the current gold standard assay. We have applied to the FDA requesting that Tulane-affiliated hospitals be allowed to use this assay for general COVID-19 diagnosis under Emergency Use Authorization regulations. My group is now validating the ability of ability of this assay to diagnose COVID-19 using other diagnostic samples that can be more readily collected than nasopharyngeal swabs used in most current assays or which can provide different diagnostic or prognostic information. We are also refining this assay approach to be read on inexpensive hand-held devices that are suitable for large scale deployment in community-based screening efforts.
Publications:
- Hu, T.*, Frieman, M.*, Walfram, J.* Chloroquine against COVID-19: insights from the field of nanomedicine. Nature Nanotechnology. 2020, 15:247-249.
- Liu, J., Wan, M., Lyon, C., Hu, T. Nanomedicine therapies modulating macrophage dysfunction: a potential strategy to attenuate cytokine storms in severe infections. Theranostics 2020, accepted.
- Niu, A., McDougal, A., Ning, B., Safa, F., Luk, A., Mushatt, D., Fusco, D., Nachabe, A., Zwezdaryk, K., Robinson, J., Socola, F., Safah, H., Hu, T., Saba, N. S. Diagnosis and Management of COVID-19 using CRISPR in allogeneic hematopoietic stem cell transplant recipients with false negative RT-PCR. Bone Marrow Transplantation 2020, in press.
- Huang, Z., Tian, D., Liu, Y., Lin, Z., Lyon, C., J., Lai, W., Fusco, D., Drouin, A., Yin, X., Hu, T.*, Ning, B.*. Ultra-Sensitive High Throughput CRISPR Diagnosis of COVID-19. Biosensor and Bioelectronics 2020. In press.
Platform II.
Nanoplasmonic quantification of pathogen-derived extracellular vesicles
Extracellular vesicles (EVs) are small membrane bound vesicles secreted by most cells and which circulate at high abundance. EVs function as carriers of genes, soluble factors and membrane-bound receptors/ligands from a parent cell/bacteria to local or distant cells, where EV uptake can alter the phenotype of the recipient cell. EVs are thus excellent candidates for biomarkers. EVs secreted by cancer cells can modulate alter the phenotype of adjacent and distant cells to promote tumor growth and metastasis. Our group has thus employed proteomics to analyze EVs derived from infected or cancerous cells to identify new diagnostic biomarkers. For example, we have used this approach to identify transmembrane proteins overexpressed on EVs derived from non-malignant versus malignant pancreatic and lung cell lines that can be utilized as biomarkers of pancreatic and lung cancer. Most current EV assays require an EV isolation step prior to analysis, which renders them impractical for most clinical applications. We have therefore developed nanoplasmonic near-field and far-field dark field microscopy assays that could directly analyze specific EV biomarker abundance from serum samples.
Publications:
- Liang K., Liu, F., Fan, J., Sun, D., Bernard, D. W., Li, Y., Katz, M. H., Koay, E. J., Zhao, Z., Hu, T. Nanoplasmonic Quantification of Tumor-derived Extracellular Vesicles in Plasma Microsamples for Diagnosis and Treatment Monitoring. Nature Biomedical Engineering. 2017, 1:0021.
- Rodrigues, M., Richards, N., Ning, B., Lyon, C., Hu, T. Rapid lipid-based normalization of membrane protein expression on extracellular vesicles in complex biological samples. Nano Letters. 2019, 19, 11, 7623-7631 (Cover Story).
- Zhao, Z., Ning, B. Lyon, C., Hu, Y. Extracellular Vesicles as Cancer Liquid Biopsies: from Discovery to Clinical Application. Lab-on-a-Chip. 2019, 19(7):1114-1140 (Cover Story).
- Liu, Y., Fan, J., Xu, T., Ahmadinejad, N., Hess, K., Lin, S., Zhang, J., Liu, X., Liu, X., Ning, B., Liao, Z., Hu, T. Extracellular Vesicle Tetraspanin-8 Expression Predicts Distant Metastasis in Non-Small Cell Lung Cancer after Concurrent Chemoradiation. Science Advances. 2020, 6:11, eaaz6162.
Platform III.
Peptidomic quantification of pathogen antigens for a personalized diagnosis
A major focus of our research is the identification of pathogen-derived biomarkers in patient serum samples. Such biomarkers are highly advantageous, since they avoid the need for invasive biopsies that may miss the site of infection or fail to reflect the degree of systemic infection. Our group employs nanomaterials and mass spectrometry to achieve the high level of analytical sensitivity and specificity required to detect target biomarkers against the abundant and diverse background of proteins present in human serum. Mass spectrometry represents an attractive means of identifying serum biomarkers derived from bacterial species, since it can discriminate peptides derived from homologous proteins, such as virulence factors, which are expressed by multiple pathogen species. We have used this approach to design the diagnostic platform employed in this proposal as well as to identify biomarkers that can distinguish clinically important non-tuberculous mycobacteria species.
Publications:
- Liu, C., Zhao, Z., Fan, J., Lyon, C. J., Wu, H., Nedelokv, D., Zelazny, A.M., Olivier, K.N., Cazares, L.H., Holland, S.H., Graviss, E. A., Hu, Y. Quantification of Circulating M. tuberculosis Antigens for Rapid Diagnosis and Real-time Treatment Monitoring. Proc. Natl. Acad. Sci. U S A. 2017, 114(15):3969-3974.
- Fan, J., Zhang, H., Nguyen, D., Lyon, C. J., Mitchell, C. D., Zhao, Z., Graviss, E. A., Hu, Y. Rapid diagnosis of new and recurrent tuberculosis by quantification of circulating antigen in HIV-infected adults in the Greater Houston Metropolitan area: a retrospective cohort study. BMC Medicine 2017, 15:188.
- Liu, C., Lyon, C. J., Deng, Z., Walters, E., Li, Y., Zhang, L., Hesseling, A., Hu, Y. Clinical evaluation of a blood assay to diagnose paucibacillary tuberculosis via bacterial antigens. Clinical Chemistry 2018, 64(7): 1 (Cover Story).
- Zhang, W., Shu, Q., Zhao, Z., Fan, J., Lyon, C. J., Zelazny, A., Hu, Y. Antigen 85B peptidomic analysis allows species-specific mycobacterial identification. Clinical Proteomics 2018, 15:1.
Platform IV.
Nanotechnology-based sensing platforms
To manufacture sensing devices with the precision and consistency required for clinical use, we applied micro-/nano-fabrication technology developed and established over the past 50 years in the semiconductor industry. Applying nanotechnology to the biosciences offers an enormous advantage due to immediate access to an array of sophisticated tools. Scalability, precision, and reproducibility are valuable characteristics of the micro-machining processes that can be translated into clinical applications. My research team has been developing integrated nanomaterial-based microsystems, semiconductor chips and nanotechnologies for imaging, sensing, and regulating cellular processes critical to healthcare, environmental, and defense applications. Nano-Micro scale science, information, and biomedicine are integrative components of my research, and are used in combination with advanced engineering tools to facilitate biomedical studies and develop robust diagnostics for global health initiatives.
Publications:
- Sun, D., Fan, J., Liu, C., Liu, Y., Bu, Y., Lyon, C. J., Hu, Y. A noise reduction method for quantifying nanoparticle light scattering in low magnification dark-field microscope far-field images. Anal. Chem. 2016, 88(24): 12001-12005.
- Sun, D., Lyon, CJ., Hu, T. A low cost mobile phone-based dark-field microscope for nanoparticle-based quantitative studies. Biosensors and Bioelectronics. 2017, 15;99: 513-518.
- Amrollahi P., Rodrigues M., Lyon, C. J., Goel, A., Han, H., Hu, T. Ultra-sensitive automated profiling of EpCAM expression on tumor-derived extracellular vesicles. Frontiers in Genetics. 2019, 10:1273.
- Sun, D., Lyon, C., Hu, T. Simulation-directed amplifiable nanoparticle enhanced quantitative scattering assay under low magnification darkfield microscope. Journal of Material Chemistry B. 2020, 8: 5416-5419.
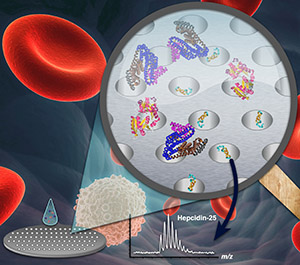
Platform V.
Nanopore-Enabled Peptidomic Analysis and Biomarker Discovery
Low molecular weight (LMW) peptides, rather than bulky and abundant proteins, are more viable sources of biomarkers; disease-associated peptides (secreted by cells, shed from their surface or otherwise released) are more likely to enter the bloodstream where we can quickly and easily survey them as part of an early detection strategy for health care. However, despite great investment of resources, efforts to identify serological protein/genetic biomarkers for most diseases have met with limited success. Most current techniques can only capture high molecular weight (HMW) proteins, whereas the real information encoded within low-abundance, small peptides remains elusive, hindering our true understanding of the immunologic process of disease malignance and our ability to detect its early stage with any real accuracy. Our research design addresses current conceptual and technical gaps. We have developed a tunable, nanopore-based platform to effectively fractionate peptides from blood samples with little to no sample processing. With the superior capillary adsorption of nanopore films to peptides via engineering of the nanopore’s physico-chemical properties (e.g., pore size, pore structure, surface affinity), we have been able to enrich these LMW peptides from serum and preserve their integrity. By coupling this platform to advanced mass spectrometry technology and customized biostatistics methods, our discoveries have enormous promise for rapid translation to the clinic, particularly by offering an inexpensive and precise platform for disease diagnosis in vulnerable populations. The examples below highlight our contribution to this field.
Publications:
- Li Y., Li Y., Chen T., Kuklina A. S., Bernard P., Esteva F. J., Shen H., Hu T. Circulating Proteolytic Products of Carboxypeptidase N for Early Detection of Breast Cancer. Clin. Chem. 2014, 60(1): 233-242.
- Wu H. J., Li Y., Fan J., Deng Z., Hu Z., Liu X., Graviss E. A., Ma X., Hu T. Antibody-Free Detection of M. tuberculosis Antigen Using Customized Nanotraps. Anal. Chem. 2014; 86 (4), pp 1988–1996.
- Wu, B., Ouyang, Z., Lyon, C. J., Zhang, W., Clift, T., Bone, C., Zhao, Z., Kimata, J., Hu, T. Plasma C4b peptide levels and HLA-B*57 genotype are associated with spontaneous HIV suppression in HIV-1-infected patients. ACS Infect. Dis. 2017, 3 (12): 880–885.
- Zhang, F., Lyon, C. J., Fan, J., Hu, T. A cathepsin B-dependent cleavage product of serum amyloid A identifies patients with chemotherapy-related cardiotoxicity. ACS Pharmacology and Translational Science. 2019, 2, 5, 333-341 (Cover Story).
- Deng, Z., Zhao, Z., Basilio, J., Ye, Y., Mann, K., Fu, J., Gu, Y., Wu, X., Chiao, P., Hu, T. Nanotrap-enabled quantification of KRAS-induced peptide hydroxylation in blood for cancer early detection. Nano Research. 2019, 12:6: 1445-1452.
- Liu, Y., Gu, Y., Lyon, C. J., Fan, J., Koay, E., Katz, M., Han, H., Von Hoff, D., Hu, T. Circulating levels of hydroxylated bradykinin function as an indicator of tissue HIF-1α expression. Science Bulletin. 2020, 65: 1570-1579.
