Tulane Researchers Discover New Way to Understand Pulmonary Fibrosis
Tulane researchers have discovered a new way to better understand—and potentially treat—pulmonary fibrosis, a serious lung disease that causes scarring and makes breathing increasingly difficult.
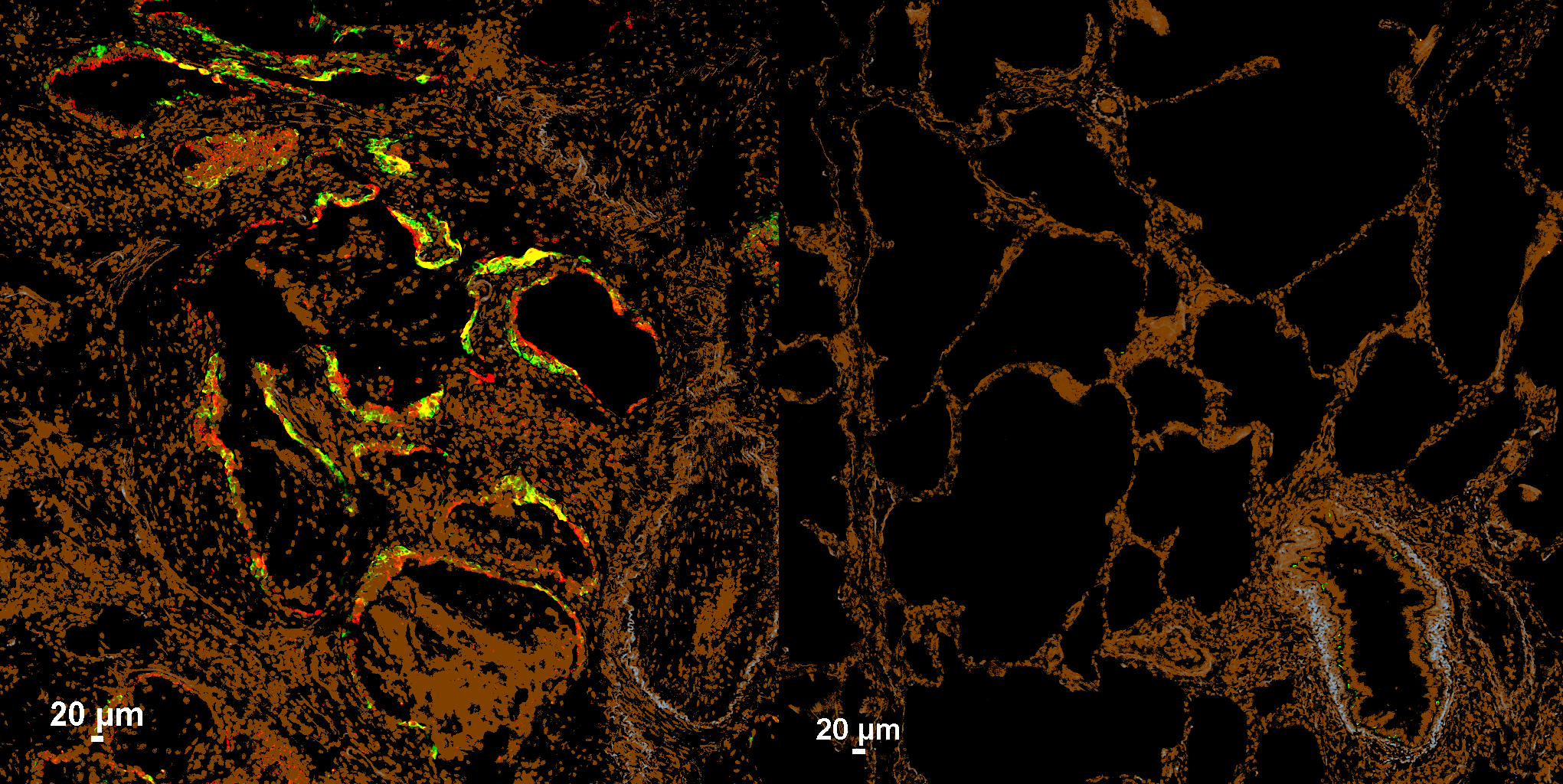
Tulane pulmonary researchers developed a precision approach to target injury-stalled alveolar stem cells and reduce lung fibrosis.
A new Nature Communications study from the laboratory of Yong Zhou, PhD, reports a transcriptomic “address label” that enables selective targeting and functional testing of a key transitional epithelial cell state implicated in pulmonary fibrosis. The work was co-led by Zhen Zheng, PhD, Assistant Professor in the Zhou laboratory and a co-first author of the study.
Pulmonary fibrosis, including idiopathic pulmonary fibrosis (IPF), is characterized by progressive scarring that stiffens the lungs and impairs breathing. Over the past several years, single-cell technologies have revealed that during injury, some alveolar epithelial stem cells move into intermediate “transitional” states. These cell states appear frequently in fibrotic lungs, but an open question has been whether they simply reflect damage or actively help drive scarring.
In this study, the Zhou laboratory developed an approach to selectively identify and manipulate one of these transitional epithelial populations in living lung tissue. The team pinpointed SPRR1A (Sprr1a in mice) as a shared marker of injury-associated intermediate epithelial cells seen in both experimental mouse fibrosis models and human IPF datasets, distinguishing them from other lung cell types.
Using this marker as a molecular “zipcode,” the researchers applied a programmable RNA-sensing strategy to turn on a reporter specifically in Sprr1a⁺ transitional cells in vivo.
They then took a crucial next step: directly testing whether these cells contribute to scarring. By implementing an RNA-guided diphtheria toxin receptor (DTR) system, the team conditionally eliminated Sprr1a⁺ transitional cells after lung injury in mice. This targeted depletion markedly reduced fibrosis, providing evidence that this transitional epithelial state can be a driver of disease, rather than merely a bystander.
Beyond the biological insight, the work demonstrates a broader concept: single-cell transcriptomic signatures can be used to guide highly selective interventions in complex tissues like the lung. The long-term implication is that future strategies might not only remove harmful cell states, but potentially reprogram them to support normal repair.
This effort reflects broad team science, with collaborations across Tulane pulmonary laboratories (Drs. Victor J. Thannickal, Joseph A. Lasky, and Shigeki Saito) and external partners at Baylor College of Medicine and University Hospital Heidelberg, Germany.
The project also received scRNA-seq support through Tulane’s NextGen Sequencing Core within the Center for Translational Research in Infection & Inflammation, including assistance from Drs. Jay K. Kolls and Kejing Song.
Publication: “Transcriptomic signature-guided depletion of intermediate alveolar epithelial cells ameliorates pulmonary fibrosis in mice.” Nature Communications. 2026 Jan 10.
doi: 10.1038/s41467-026-68354-y. Online ahead of print (https://www.nature.com/articles/s41467-026-68354-y)