Unraveling the Mechanisms Underlying Telomere Maintenance in Drosophila
We are delighted to share our recent publication in Science Advances, which uncovers key molecular mechanisms regulating telomeric retrotransposon (TR) transcription in Drosophila melanogaster. This study was led by Dr. Mengmeng Liu in the Ji laboratory at Tulane University School of Medicine.
Telomeres, the protective caps at the ends of chromosomes, are crucial for genome stability and replication. While most eukaryotes rely on telomerase to maintain their telomeres, Drosophila and many dipteran insects have evolved a unique strategy. Instead of telomerase, they use three telomere-specific retrotransposons—HeT-A, TART, and TAHRE (collectively known as TRs)—to elongate telomeres via retrotransposition. Despite their essential role, the mechanisms controlling TR transcription have remained unknown until now.
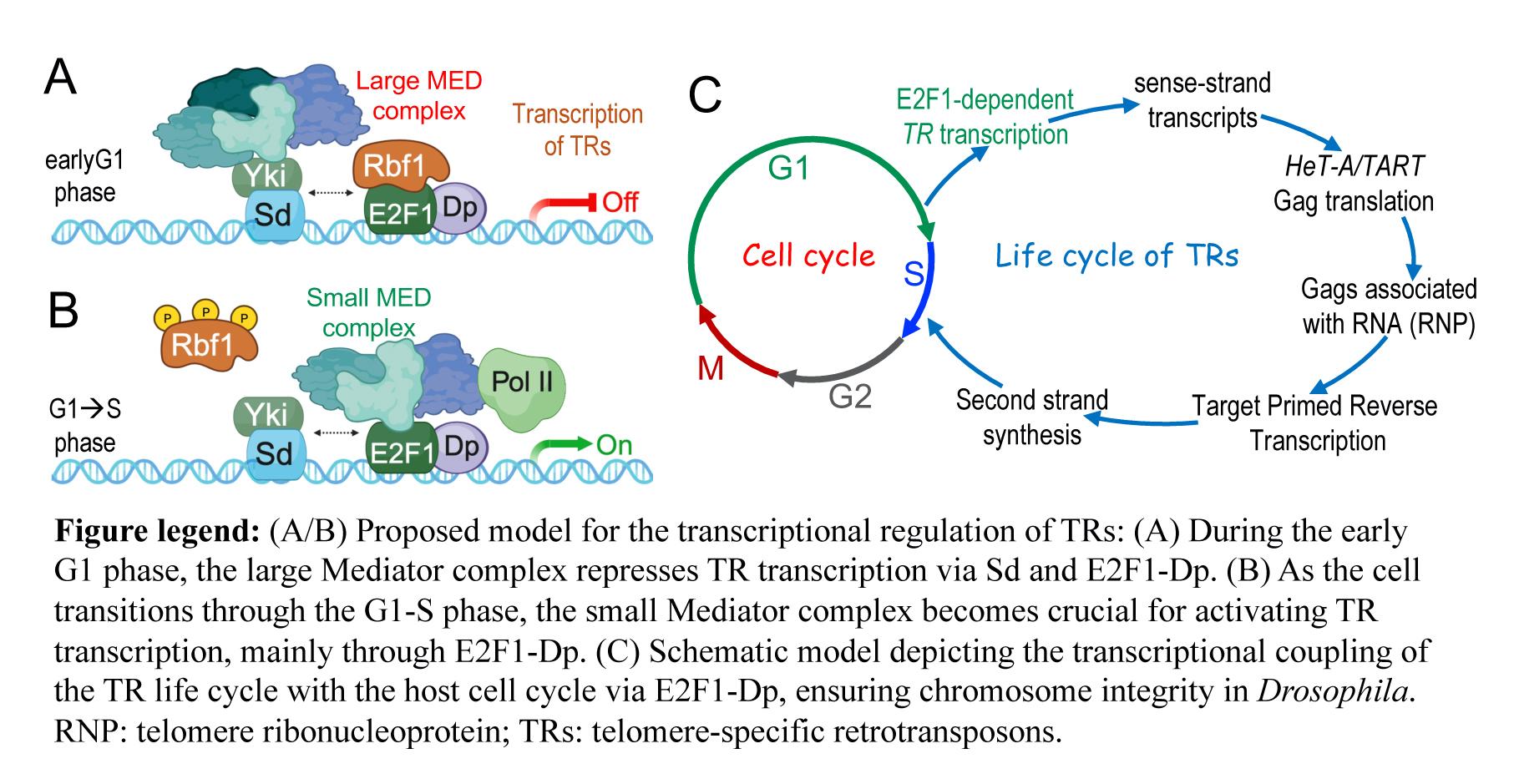
Our study reveals how Drosophila regulates TR transcription to maintain telomere stability, identifying three key regulators: (1) Mediator Complex: A multi-protein assembly that modulates gene transcription. Mutations in Mediator subunits increase TR transcription and telomere length. (2) E2F1-Dp: A cell-cycle regulator that stimulates TR transcription when overexpressed and reduces it when mutated or depleted, linking telomere dynamics to cell-cycle machinery. (3) Scalloped (Sd)/dTEAD: A transcription factor that collaborates with the Mediator complex and E2F1-Dp to suppress TR transcription. Using CUT&RUN analysis, we demonstrated that CDK8 (a Mediator subunit), Dp, and Sd/dTEAD directly bind to TRs. Motif enrichment analysis further revealed E2F and TEAD binding sites, supporting the specificity of these interactions. These findings reveal an exceptional aspect of telomere maintenance strategy in Drosophila, providing a framework for understanding alternative mechanisms in different species. By coupling TR transcription to host cell-cycle machinery, Drosophila achieves robust telomere regulation without telomerase.
Our research highlights a fascinating alternative to the telomerase-based system seen in most species. It demonstrates how Drosophila has evolved a unique and robust approach to maintain chromosome ends, directly linking this process to its cell cycle. This discovery deepens our understanding of the creativity of evolution in solving essential problems like maintaining chromosome integrity. Exploring these alternative telomere maintenance strategies broadens our appreciation of the diversity, complexity, and richness of life on Earth.