Dr. Yi-Ping Li’s Lab Uses Animal Models to Study the Pathogenesis of Human Diseases at Tulane
Major Research Interests
We have defined ATP6i as T cell activation regulator and Cathepsin K as dendritic cell maturation regulator and as a tumor suppressing gene and its role of mutation in prostate cancer metastasis. We have developed AAV mediated gene knockdown system for therapeutic alternatives for human breast cancer bone metastasis. We have also demonstrated that whole-body Ctsk knockdown or a small molecule inhibitor of Ctsk enhance the efficacy of immunotherapy by improving anti-tumor immunity and promoting dendritic cell maturation, and that osteoclast proton pump regulator Atp6v1c1 enhances breast cancer growth by activating the mTORC1 pathway and bone metastasis by increasing V-ATPase activity.
We have identified a remarkable new drug that powerfully acts against tumors, while circumventing the difficulties and adverse effects of conventional antitumor treatments. We synthesized a novel VDA, referred to as Erianin disodium phosphate (Erianin-DP), which has a low effective dose, no major adverse effects on the cardiovascular and respiratory systems, a dramatic inhibitory effect on tumor growth in mice bearing diverse tumors, and a synergistic inhibitory effect when combined with other drugs. We are currently developing this drug further and investigating the molecular mechanism of its antitumor effects as shown in the patent list section.
The proposed study will provide important insights into the negative regulation of the cells of the immune system to target inflammation and bone destruction in RA by elucidating the underlying mechanism of Gα13 signaling. Knowledge gained from this study may generate potential therapeutic means for treating RA and other inflammatory bone diseases. We also characterizing the mechanism by which endogenous negative regulators of osteoclasts control bone homeostasis under physiological and pathological conditions in mouse models. This study endeavors to understand how osteoclast (OC) differentiation is negatively regulated and to characterize the mechanism by which endogenous negative regulators of osteoclasts control bone homeostasis under physiological and pathological conditions.
Investigating stem cell reprogramming for cartilage, bone, and tissue regeneration.
We are currently investigating if gene profile reprogrammed novel induced pluripotent stem cells (iPSCs), utilizing a lentiviral vector containing iPS transcription factors and a LoxP site which can circumvent iPS genes insertional tumorigenesis, seeded on scaffolding biomaterial will mimic natural osseous tissue generation and enable improved cartilage and bone regeneration. We integrate novel iPSC-mediated cell therapy and gene therapy via tissue master regulators gene expression for inflammation diseases, including Rheumatoid arthritis and periodontal disease and bone diseases, including osteoporosis in animal models. We have already identified several key transcription factors that will be used; we pinpointed Atp6v0d2 as a triple regulator and potentially an ideal therapeutic target for inflammation and Osterix (Osx) as a key osteogenic transcription factor to promote bone formation.
We have characterized the functions of CNBP (also known as Znf9) in head formation in chicken and mouse models through gene knockout misexpression and tissue-specific targeted disruption approaches. We also characterized Znf9+/- mice and found that their phenotype reflects many of the features in myotonic dystrophy. Znf9 is highly expressed in skeletal and heart muscles. Our data demonstrated that Znf9 haploinsufficiency contributes to the myotonic dystrophy phenotype in Znf9+/- mice. We will expand our understanding of the role of CNBP in of the biological and molecular basis of craniofacial morphogenesis and muscle development and diseases.
Although our previous studies have revealed the negative regulation of Gα13 on osteoclastogenesis and bone resorption, little is known about the function of Gα13 under physiological aging and pathological periodontitis. We are currently studying the essential function of Gα13 on reducing bone resorption and inflammation in the periodontal area during aging, and overexpression of Gα13 through local delivery could efficiently treat aging-associated periodontitis. We have revealed the important role of Gα13 in periodontal bone regeneration and inflammation during aging. This innovative work will provide strong foundations for potential treatment to dramatically improve the oral health of those struggling with periodontal disease while mitigating the effects of these local infections on various systemic diseases triggered by inflammation. This study endeavors to facilitate the design of novel therapeutic approach for periodontal and osteolytic diseases. The findings of the study will further our understanding of negative regulatory signals in inflammation response and osteoclasts activity. This study endeavors to understand the mechanisms underlying how Gα13 signaling regulates chronic inflammation and bone loss in aging-associated periodontitis. The proposed study will provide important insights into the regulation of bone resorption and inflammation in aged-associated periodontitis and inhibiting inflammation and bone erosion in periodontal disease by targeting cell endogenous negative signaling.
Our Lab currently employs novel means that will simultaneously prevent tissue damage and bone loss by reducing the inflammation and bone resorption caused by oral infectious and inflammatory diseases. We have applied our findings in osteoclast biology to translational medicine through a novel AAV mediated gene silencing tool targeting crucial osteoclast proteins (e.g. ATP6i, RGS10, C1, AC45 and Cathepsin K), which have the capacity to reduce bone loss and inflammation associated with oral diseases in mouse models of periodontal and endodontic diseases. This groundbreaking work investigates the therapeutic potential of targeting ATP6i, RGS10, and Cathepsin K as treatments for immune-mediated oral inflammatory diseases and has led to numerous insights on the roles of these genes in Osteoimmunology as treatments for immune-mediated oral inflammatory diseases. Importantly, this work has shown that a number of critical osteoclast genes also have important roles in the immune system regulation to inflammatory diseases.
My lab has investigated the pathogenesis of cleidocranial dysplasia (CCD) and revealed that CCD may result from functional defects of the Runx2/Cbfβ heterodimeric complex in various skeletal cells. My lab has characterized the mechanism by which Cbfβ regulates chondrogenesis and osteoblastogenesis and revealed that Cbfβ controls the balance of chondrocyte proliferation and differentiation by upregulating Indian hedgehog (Ihh) expression and inhibiting parathyroid hormone-related protein receptor (PPR) expression in postnatal cartilage and bone formation. My lab has also revealed the indispensable function of Cbfβ in chondrocyte maturation, growth plate development, and trabecular bone formation in mice. We revealed that Cbfβ is key osteoblast transcription factor that control osteoblast differentiation, cell lineage commitment in bone hemostasis and bone diseases e.g. osteoporosis and we have shown that the Runx/Cbfβ complex promotes Wnt10b/β-catenin signaling and inhibits C/EBPα expression to inhibit lineage switch into adipocytes.
Our lab has characterized the mechanism by which Cbfβ regulates chondrogenesis in skeletal development and revealed that Cbfβ controls the balance of chondrocyte proliferation and differentiation by upregulating Indian hedgehog (Ihh) expression and inhibiting parathyroid hormone-related protein receptor (PPR) expression in postnatal cartilage and bone formation; my lab has also revealed the indispensable function of Cbfβ in chondrocyte maturation, growth plate development, and trabecular bone formation in mice. Recently, we revealed that Cbfβ and RUNX1 are key transcription factors that control chondrocyte differentiation and cartilage repair in osteoarthritis. To develop a safer and more effective therapeutic approach to cure aging-associated osteoarthritis (OA), we found that Cbfβ mediates articular cartilage regeneration and repair in aging. This study endeavors to develop a safer and more effective therapeutic approach to cure aging-associated osteoarthritis (OA). The specific goal of this study is to characterize the mechanism underlying how core-binding factor beta (Cbfβ) mediates articular cartilage regeneration and repair in aging-associated OA
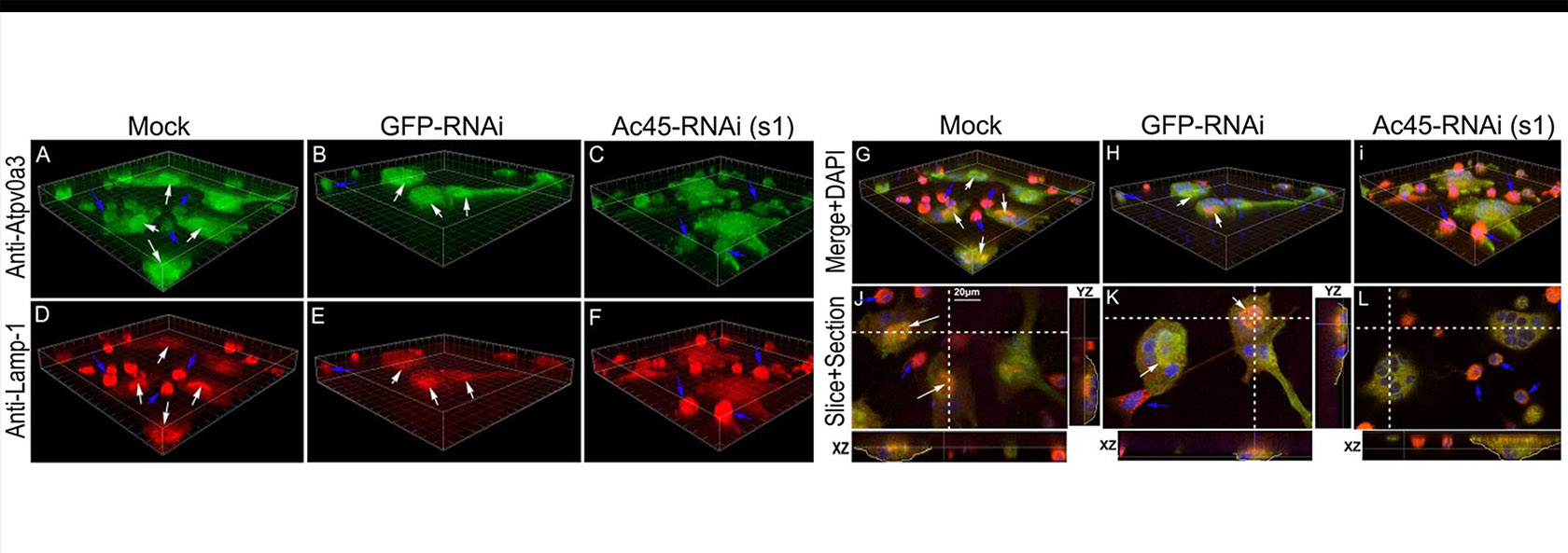
I was the one of the scientists who applied molecular biology approaches to the study of osteoclasts. My laboratory laid down part of the foundation of modern molecular osteoclast biology and revealed the molecular mechanism of osteoclast bone resorption. My Lab’s work resulted in the publication of a number of seminal papers on the cloning and characterization of the osteoclast proton pump subunits genes critical to osteoclast mediated acidification in bone resorption, including a3 or ATP6i, d2 or ATP6V0D2, C1 or ATP6V1C1, and Ac45 or ATP6AP1. We found that knockout or knockdown of these OC proton pump subunits highly expressed in osteoclasts resulted in defects of osteoclast function due to impaired acidification function of OC pump. We demonstrated that inactivation of subunit ATP6i leads to osteopetrosis in mice because of nonfunctional osteoclasts that are incapable of acidifying the extracellular resorption lacuna. My lab work resulted in the discovery and publications of the papers on the cloning and function characterization of osteoclast protease genes critical to osteoclast mediated matrix protein degradation in bone resorption, including Cathepsin K. Recently my lab found that these osteoclast function genes play critical roles in the progression of osteolytic inflammation diseases.
We characterized the response of osteoclast signaling pathways to RANKL and have determined the essential role of a number of osteoclast signaling regulators that regulate osteoclast differentiation and function, including, RGS10, RGS12, TANK, and Gα13. We revealed the role of RGS10 as a critical regulator in the RANKL-evoked RGS10/calmodulin-PLCγ-[Ca2+]i oscillation-NFATc1 signaling pathway for osteoclast differentiation. We revealed that Gα13 is a master endogenous negative switch for osteoclastogenesis through regulation of the RhoA/Akt/GSK3β/NFATc1 signalling pathway. Recently, we found the osteoclast signaling regulators play key role in in the progression of osteolytic inflammation diseases, including osteoarthritis and rheumatoid arthritis. Importantly, our recent work has shown that a number of critical osteoclast genes, including ATP6i, RGS01, Cathepsin K and Gα13 also are important immune response regulators in oral diseases and Rheumatoid arthritis.
We have determined the essential role of a number of osteoclast transcription factors that control osteoclast differentiation, cell lineage commitment, osteoclast activation and function. To characterize the mechanism by which transcription factors regulate OC lineage commitment, we mapped the critical cis-regulatory element in the promoter of cathepsin K (Ctsk), which is expressed specifically in OCs, and found that CCAAT/enhancer binding protein α (C/EBPα) is the critical cis-regulatory element binding protein. Our results indicate that C/EBPα is highly expressed in the early and late stages of OC differentiation. Recently, we revealed that RUNX1 is another key osteoclast transcription factor that controls osteoclast differentiation, activation, and function, as well as cell lineage commitment. The study of Cbx3 function in osteoclasts will elucidate the mechanism(s) by which how Cbx3 cooperating with epigenetic factor negatively regulates osteoclast (OC) differentiation. Insights gained from this study not only will address the basic question about how gene expression is regulated in OC biology, but also will provide foundation for facilitating the design of safer and novel therapeutic approach for osteolytic diseases.