The Clinical Trials content type is the required format for promoting new or ongoing clinical trials on the School of Medicine website. Using this content type ensures that trials are automatically included in our searchable Clinical Trials Directory, making them easier for patients and researchers to find. Approved content editors can create and publish clinical trials using this standardized template.
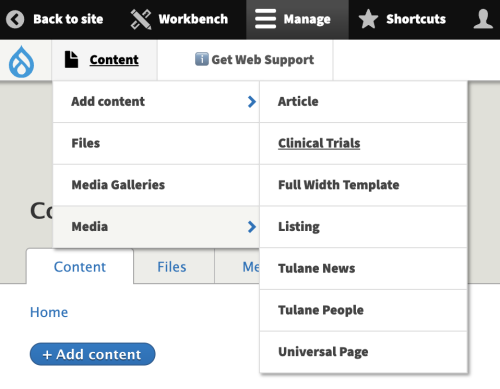
- Click Manage on the top black nav menu
- Click Content on the white sub-nav menu
- Click +Add Content at the top
- Click on Clinical Trial.

Page Content
Each Clinical Trial page is organized into four main sections to ensure that both potential participants and researchers can easily access the information they need:
Intro Section
This top section provides a brief, plain-language summary of the trial, key enrollment details (such as age range, gender, and whether healthy volunteers are accepted), and contact information for questions or assistance. This section is designed for quick understanding and outreach.
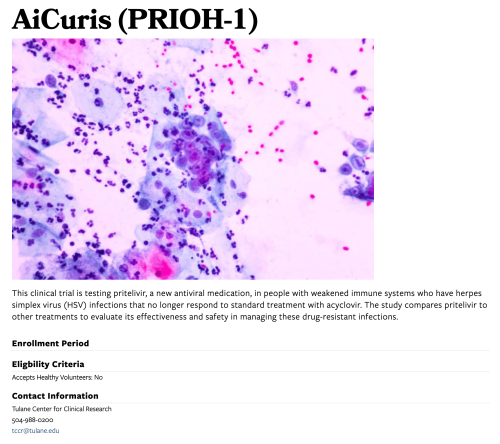
Title
Enter the title of the clinical trial. This will appear as the main heading on the published page.
Image
Upload a relevant image for the clinical trial (if no image is provided, it uses the default image).
Brief Summary
Write a short, plain-language summary of the trial. Avoid technical jargon and aim for clarity for a general audience.
Enrollment Period
Specify the start and end dates for when participants can enroll in the study.
Selection Criteria
- Gender - Select the gender(s) eligible for the study.
- Age Range - Select one or more applicable age categories.
- Accepts Healthy Volunteers - Check this box if the trial accepts participants who are not currently diagnosed with the condition being studied.
Contact Information
- Contact Name - Enter the primary contact name
- Contact Phone - Enter the contact phone number in this format: xxx-xxx-xxxx (e.g., 504-988-0200).
- Contact E-mail - Enter the email address for inquiries (e.g., tccr@tulane.edu).
Study Overview
This participant-focused section includes details about the study (Body), conditions or diseases being studied, the hosting department or organization, and a pre-filled FAQ block. The FAQ section appears on all Clinical Trial pages and includes general information to help potential participants understand what to expect when joining a clinical trial.

Body
Use this section to provide additional study details for participants.
Conditions / Diseases
List the conditions or diseases being studied. Use terms that are searchable and patient-friendly.
Department / Organization
Select the department, center, or organization sponsoring the trial (e.g., Tulane Cancer Center, Dermatology).
Researcher View
This section offers more technical, in-depth information intended for providers and research professionals. It includes details such as the trial sponsor and a link to the official listing on ClinicalTrials.gov. Content editors can use this space to elaborate on study design, methodology, or other relevant data.
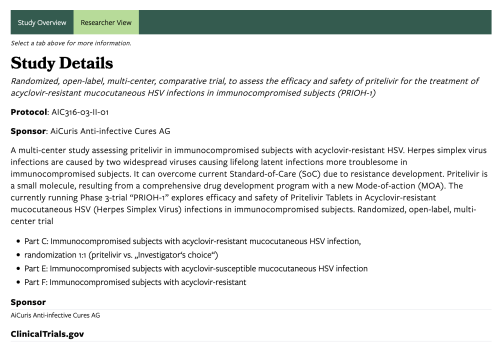
ClinicalTrials.gov Link
Provide a direct external link to the study’s official page on ClinicalTrials.gov.
Sponsor
Enter the name of the sponsor funding or managing the study.
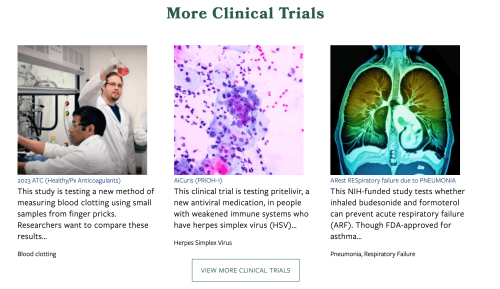
Call to Action (CTA) + Related Trials
At the bottom of every Clinical Trial page is a standardized Call to Action area with a customizable button. The button can be linked to a registration form, enrollment portal, or any relevant page to prompt user action (e.g., “Sign Up Today”). Below the CTA, a dynamic thumbnail gallery of other clinical trials displays to encourage further exploration and awareness.

Enrollment Link
URL
Provide a link to the enrollment form or study page. You can use an internal or external URL.
Link Text
Use a clear call to action. This will appear near the bottom of the page as a blue callout box.
Page Metadata
Before Publishing: Set the Menu Settings
Once all required fields have been completed, content editors must enable the Menu settings in the right-hand sidebar before publishing the Clinical Trial page.
To do this:
- Click the checkbox labeled “Provide a menu link.”
- Enter a shortened version of the trial title in the Menu link title field. This will appear in the site’s sidebar navigation.
- The Parent link should automatically default to “Clinical Trials.” If not, be sure to select it from the dropdown.
This step ensures the trial is properly linked within the Clinical Trials section of the site and can be easily navigated by users.
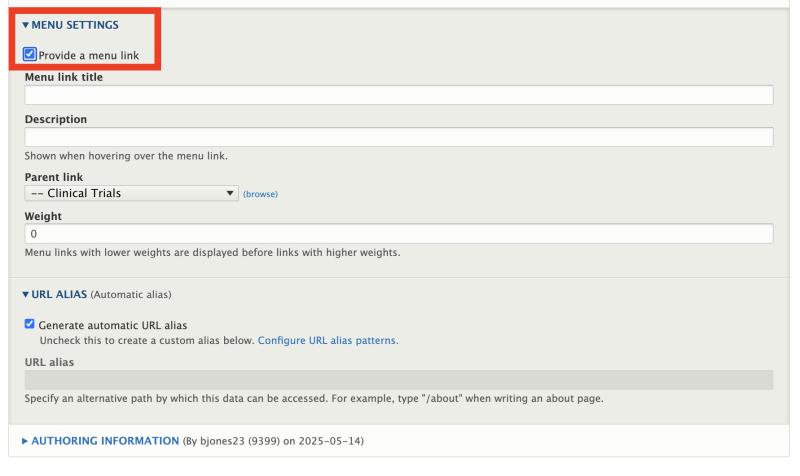
After selecting the menu link, you may then proceed to saving/publishing the clinical trial. All clinical trial URLs will default to /clinical-trials/active/<title-of-your-trial>.
Need Help or Have Feedback?
If you have questions or need help using this content type, you can:
👉 Sign up for a one-on-one training session with the web designer
📝 Submit feedback to help improve the editing experience
