No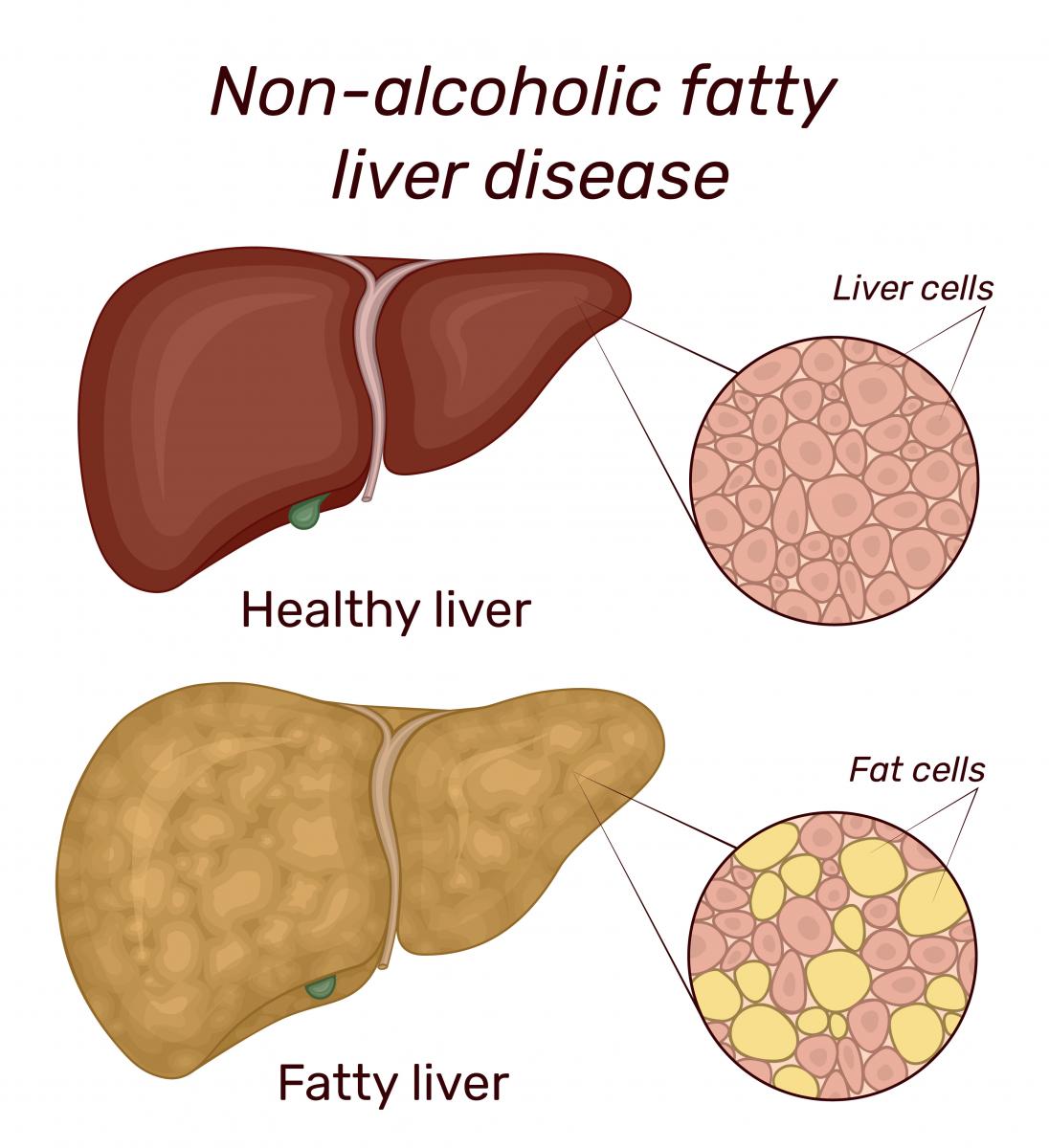 n-alcoholic fatty liver disease (NAFLD) is a chronic liver disease in which too much fat is stored in the liver. Normally, there are no symptoms, and the condition is discovered during blood tests or other diagnostic tests. As the name implies, it is not caused by alcoholism or alcohol abuse. The most common risk factors of NAFLD are obesity, gastric bypass surgery, high cholesterol, and type 2 diabetes. If the disease progresses, it can lead to non-alcoholic steatosis (NASH).
n-alcoholic fatty liver disease (NAFLD) is a chronic liver disease in which too much fat is stored in the liver. Normally, there are no symptoms, and the condition is discovered during blood tests or other diagnostic tests. As the name implies, it is not caused by alcoholism or alcohol abuse. The most common risk factors of NAFLD are obesity, gastric bypass surgery, high cholesterol, and type 2 diabetes. If the disease progresses, it can lead to non-alcoholic steatosis (NASH).
NASH is a severe form of NAFLD. It is the most common form of liver disease in the United States. Like NAFLD, there are usually no symptoms. In NASH, the liver is inflamed and after years of inflammation, the liver can develop scar tissue or “fibrosis”. Severe fibrosis is called cirrhosis.
The liver research team, part of Tulane's Section of Gastoenterology and Hepatology, contributes to the research of fatty liver disease (NASH and NAFLD). We are currently enrolling volunteers for the following studies:
Lakeside Life Sciences LLS-019: Evaluation of the diagnostic performance of the M30 Apoptosense® M65 and M65 EpiDeath ELISA Assay results to stratify non-alcoholic steatohepatitis (NASH) from simple steatosis in a cohort of patients suspected of nonalcoholic Fatty Liver Disease (NAFLD) (PI: Dr. Ting)
If you would like more information about liver disease, call 504-988-5344.
If you are interested in participating in research, please contact us at or 504-988-3047 or click here to request additional information
Pr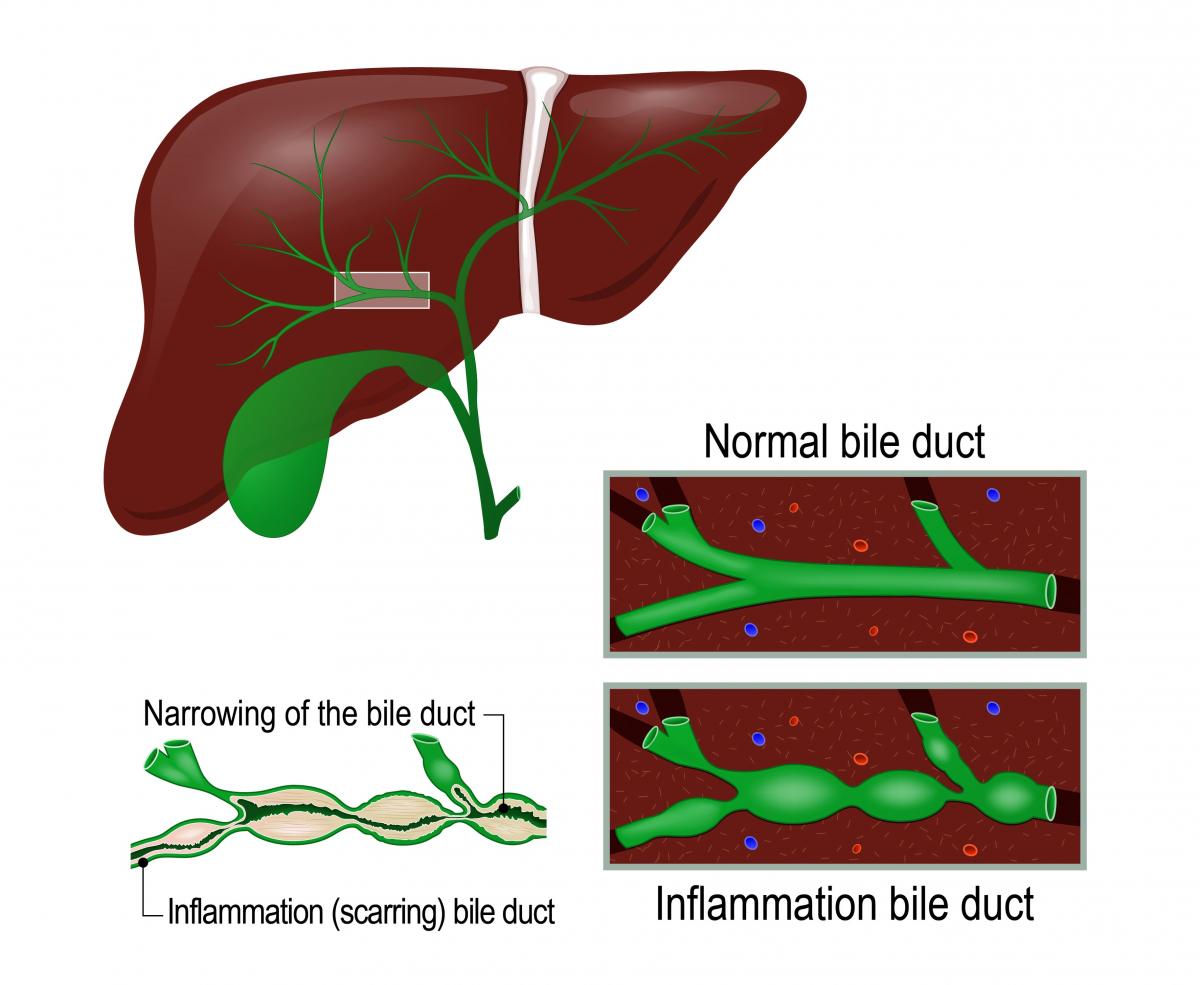 imary biliary cholangitis (PBC) is a chronic disease that affects the liver. In PBC, the bile ducts of the liver become damaged, causing bile to back up in the liver which can lead to scarring (cirrhosis). The cause of PBC is unknown, but it is most likely an autoimmune disease and probably genetic. Often, there are no symptoms, but common early symptoms include fatigue, itchiness (pruritus), and in severe cases, jaundice.
imary biliary cholangitis (PBC) is a chronic disease that affects the liver. In PBC, the bile ducts of the liver become damaged, causing bile to back up in the liver which can lead to scarring (cirrhosis). The cause of PBC is unknown, but it is most likely an autoimmune disease and probably genetic. Often, there are no symptoms, but common early symptoms include fatigue, itchiness (pruritus), and in severe cases, jaundice.
Currently, there is no cure for PBC, but medication can manage the disease and slow the progression of liver damage. There is also promising research for medication to treat and manage PBC.
The liver research team, part of Tulane's Section of Gastroenterology and Hepatology, contributes to the research of PBC. We are currently enrolling volunteers for the following studies:
Escient EP 547-201: A clinical research study to investigate EP547 for patients with itch associated with liver disease (PI: Dr. Martin Moehlen) NCT05525520
The EP 547-201 study is testing an investigational drug (EP547) to see if it can help reduce itching, also known as pruritus, in adults with cholestatic liver disease.
You may be able to participate in the PACIFIC study if all the following criteria are met:
- You are 18 years of age or older.
- You have daily or near-daily moderate to severe itching (pruritus) due to PBC or PSC.
- If currently taking medications to treat liver disease or pruritus, you must be on a stable dose for certain period of time prior to study entry and willing to maintain a stable regimen throughout the study.
For more information about diagnosis or treatment options please call or 504-988-5344
If you are interested in participating in research, please contact us at 504-988-3047 or click here to request additional information.
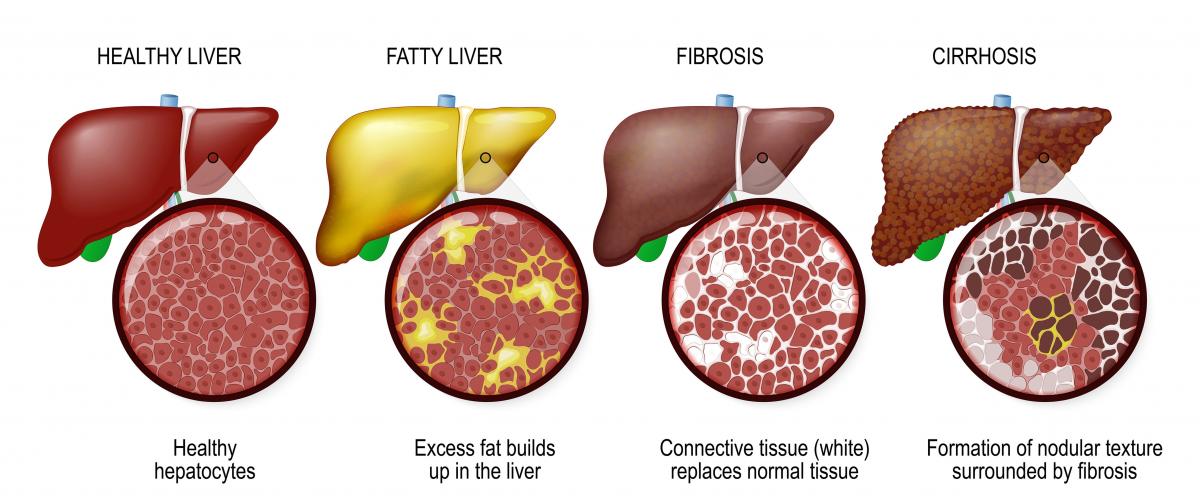
Cirrhosis is a liver condition in which scar tissue has replaced liver tissue after years of damage. Diseases that may lead to cirrhosis include fatty liver disease, hepatitis B, bile duct diseases (primary biliary cholangitis and primary sclerosing cholangitis), genetic disorders, and autoimmune hepatitis. But the most common causes of cirrhosis are hepatitis C and alcohol-related disease. In the early stages, there are few if any symptoms, but as the disease progresses, symptoms may include abdominal pain, loss of appetite, weight loss, itching and fatigue. In later stages, there may be jaundice (yellowing of the eyes and skin), bruising, and swelling of the abdomen and legs.
Treatment of cirrhosis begins with treating the underlying cause of liver damage in order to prevent further damage. For instance, if the cause of cirrhosis is fatty liver disease, weight loss and diet may prevent further damage. If the cirrhosis is the result of hepatitis B or C, the virus can be treated with medication.
In later stages of cirrhosis, liver transplant may be an option.
New medications are being developed to manage cirrhosis, and the liver research team, part of Tulane's Section of Gastoenterology and Hepatology, contributes to the research of liver cirrhosis. We are currently enrolling volunteers for the following studies:
Salix RNLC3131:A randomized, double-blind, placebo-controlled, multi-center study to assess the efficacy and safety of Rifaximin soluble solid dispersion (SSD) tablets for the delay of encephalopathy decompensation in cirrhosis (PI: Dr. Fredric Regenstein) NCT05071716
Nearly half of people living with cirrhosis get hepatic encephalopathy (HE). HE is a complication of cirrhosis that can cause symptoms like:
- Confusion and personality changes
- Loss of small hand movements
- Tremors in hands and arms
- Sleep problems
The RNLC3131 Study is looking to see if a study medicine, rifaximin, can safely delay or prevent HE in adults with advanced liver cirrhosis. You may be eligible to participate if you are between the ages of 18 and 85 and have been diagnosed with liver cirrhosis.
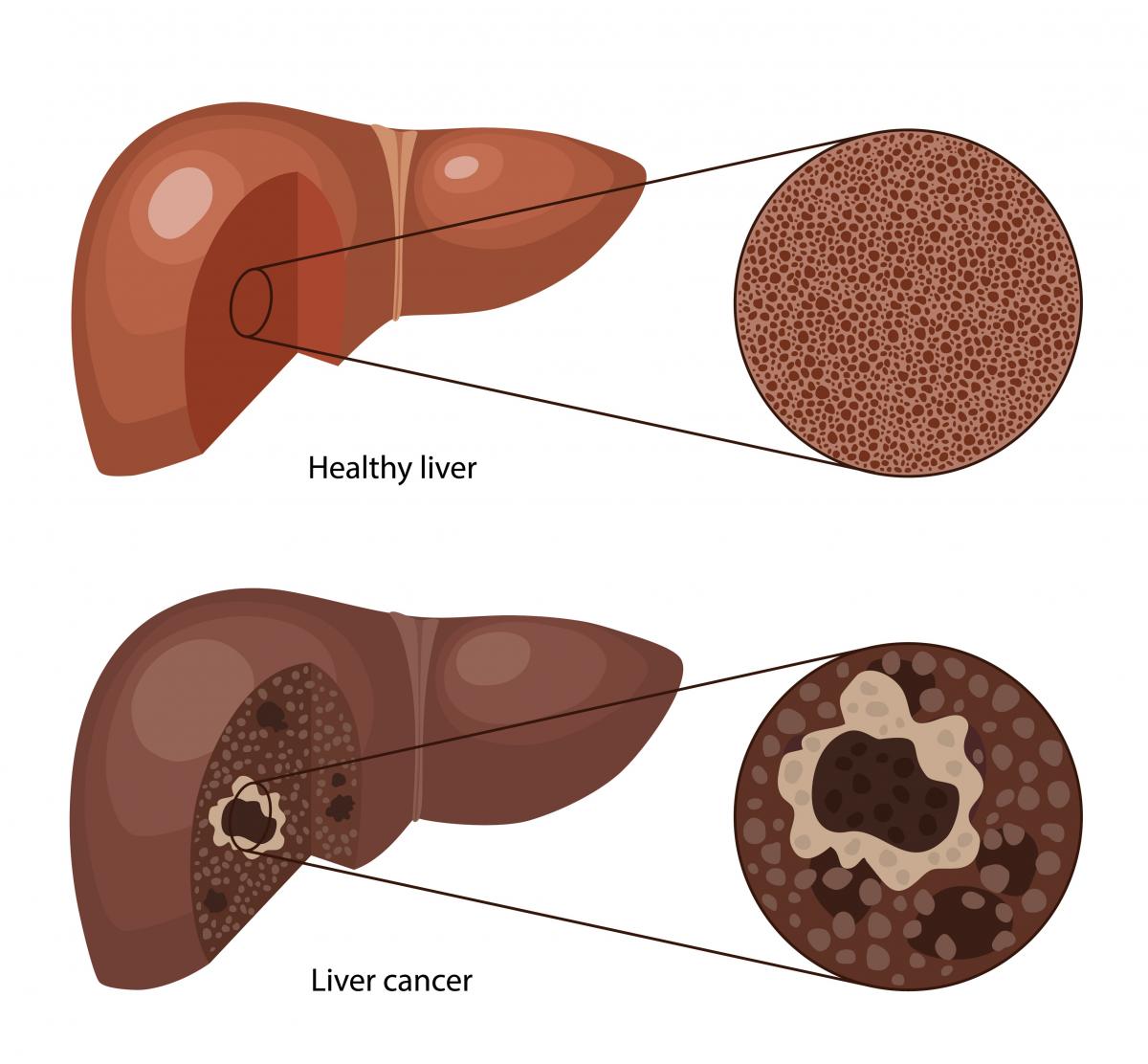 Hepatocellular carcinoma (HCC) is a primary cancer of the liver. HCC is rare in the general population but is unfortunately common in patients with severe scarring of the liver (cirrhosis) from any cause. HCC is also common in patients with longstanding chronic hepatitis B without cirrhosis. Liver tumors can often be diagnosed confidently with non-invasive radiology scans (ultrasound, CAT scans, or MRI) and no biopsy. HCC is a particularly deadly cancer if not treated aggressively. Small cancers can be treated with minimally invasive techniques or a liver transplant. Larger tumors or cancers that have spread outside the liver (metastatic) are generally not curable. Incurable tumors can be palliated with very targeted, minimally invasive therapy or oral chemotherapy.
Hepatocellular carcinoma (HCC) is a primary cancer of the liver. HCC is rare in the general population but is unfortunately common in patients with severe scarring of the liver (cirrhosis) from any cause. HCC is also common in patients with longstanding chronic hepatitis B without cirrhosis. Liver tumors can often be diagnosed confidently with non-invasive radiology scans (ultrasound, CAT scans, or MRI) and no biopsy. HCC is a particularly deadly cancer if not treated aggressively. Small cancers can be treated with minimally invasive techniques or a liver transplant. Larger tumors or cancers that have spread outside the liver (metastatic) are generally not curable. Incurable tumors can be palliated with very targeted, minimally invasive therapy or oral chemotherapy.
The liver research team, part of Tulane's Section of Gastoenterology and Hepatology, contributes to the research of liver cancer (HCC).
For more information about diagnosis or treatment options please call 504-988-5344
If you are interested in participating in research, please contact us at or 504-988-3047 or click here to request additional information.
 Hepatitis B is a viral infection of the liver. In acute infection patients may experience nausea, vomiting, jaundice (yellowing of the skin), and abdominal pain. Infection in adults is usually acquired through sexual contact or needle sticks. Most adults will clear the acute infection and be protected from hepatitis B infection thereafter. A small percentage of patients will remain chronically infected with the virus remaining in their blood and liver indefinitely. Chronic infection can lead to severe liver scarring and liver failure (cirrhosis). It also puts the patient at higher risk of liver cancer (hepatocellular carcinoma). Young children may become chronically infected during the birth process or through household exposure. Infected children do not often acquire the acute symptoms listed above; however, their lifetime risk of cirrhosis and cancer is high if not treated. Treatment of chronic hepatitis B can suppress the virus and decrease the risk of chronic liver damage. We specialize in the individualized treatment of chronic HBV and offer clinical trials that are advancing the field. Patients are often followed with occasional blood tests. A liver biopsy is occasionally recommended to guide treatment.
Hepatitis B is a viral infection of the liver. In acute infection patients may experience nausea, vomiting, jaundice (yellowing of the skin), and abdominal pain. Infection in adults is usually acquired through sexual contact or needle sticks. Most adults will clear the acute infection and be protected from hepatitis B infection thereafter. A small percentage of patients will remain chronically infected with the virus remaining in their blood and liver indefinitely. Chronic infection can lead to severe liver scarring and liver failure (cirrhosis). It also puts the patient at higher risk of liver cancer (hepatocellular carcinoma). Young children may become chronically infected during the birth process or through household exposure. Infected children do not often acquire the acute symptoms listed above; however, their lifetime risk of cirrhosis and cancer is high if not treated. Treatment of chronic hepatitis B can suppress the virus and decrease the risk of chronic liver damage. We specialize in the individualized treatment of chronic HBV and offer clinical trials that are advancing the field. Patients are often followed with occasional blood tests. A liver biopsy is occasionally recommended to guide treatment.
For more information please call 504-988-5344.
Chronic hepatitis C is a viral infection (HCV) which can lead to serious liver disorders such as cirrhosis and liver cancer. Today HCV is one of the leading causes of liver failure requiring transplantation in the United States. Despite what is commonly written in the media, HCV is curable in many patients. Currently treatments involve antiviral medications (DAA's) for 8 to 12 weeks. The Section of Gastroenterology and Hepatology was involved in the cutting edge research leading to the FDA approval of currently used HCV therapies. The late world renowned hepatologist and gastroenterologist, Luis Balart, MD, MACG describes in the video below the HCV research that was taking place within the Section.
For more information please call 504-988-5344.
